Result Analysis
Assignment:0 Prerequisite
NOTE:
Note: subject of the mail should be start with the Enrollment no.
(e.g 140190105--- Word file for oc&UP)
Marking system of OEP
Some students did not complete their submission in the subject OC&UP on the date 03/11/2015 they can do it on next day.
Assignment:0 Prerequisite
- Write 50 IUPAC names of organic molecules and draw their structures.
- Draw 50 structures of organic molecules and write their IUPAC names.
Assignment:1 Expected date of submission(07-08-2015) [in any form soft or hard(hand written)]
1. Differentiate fission and fusion reaction with two examples.
2. Define the terms : Free Radical, Carbonium, Carbanion, Carbenes and Nitrenes. Nucleophilles and Electrophilles with suitable example and figure.
3. What are SN1 and SN2 reactions explain with examples one of each.
4. Describe four main types of organic reactions.
5. Define Optical, Geometrical and Conformational Isomerism with proper examples.
6. What is call optical activity with respect to organic compounds?
7. Explain Polarimeter with its instrumentation, working and importance.
8. Define specific rotation with importance.
9. Explain the concept of Diasteromers, Enantiomers and meso compound with the example of lactic acid and tartaric acid.
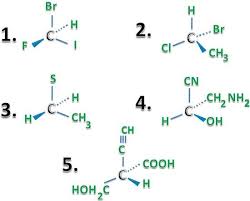
10. Finde out the absolute configuration of followings.
11. Explain E and Z designation with two examples.
12. How would you carried out Resolution of (i) racemic mixture of acids and (ii) racemic mixture of bases?
Assignment:2
1. Differentiate fission and fusion reaction with two examples.
2. Define the terms : Free Radical, Carbonium, Carbanion, Carbenes and Nitrenes. Nucleophilles and Electrophilles with suitable example and figure.
3. What are SN1 and SN2 reactions explain with examples one of each.
4. Describe four main types of organic reactions.
5. Define Optical, Geometrical and Conformational Isomerism with proper examples.
6. What is call optical activity with respect to organic compounds?
7. Explain Polarimeter with its instrumentation, working and importance.
8. Define specific rotation with importance.
9. Explain the concept of Diasteromers, Enantiomers and meso compound with the example of lactic acid and tartaric acid.
10. Finde out the absolute configuration of followings.
12. How would you carried out Resolution of (i) racemic mixture of acids and (ii) racemic mixture of bases?
Assignment:2
- Differentiate between unit process and unit operation Discuss the advantages of continuous process and batch process over each other
- Define nitration. Enlist various nitrating agents and discuss the function of sulphuric acid in the mixed acid used for nitration
- Describe the batch nitration of benzene with mixed acid Give an account on methods of reduction for the preparation of amines. Illustrate various products of reduction of nitrobenzene
- Explain kinetics and mechanism of aromatic nitration. What is the effect of Nitrous acid on nitration
- Describe the manufacture of p – nitro acetanilide Define unit process. Why is it so called? How do unit processes differ from unit operation?
- Give a brief account of nitration of paraffinic hydrocarbon Give evidence to establish the formation of nitryl ions in a nitration process conducted using mixed acids
- Describe following unit process –with suitable industrial important chemicals:Amination, Hydrogenation, Halogenations, Oxidation, Reduction, Sulphonation, Hydrolysis, Alkylation and Polymerization
- Explain the manufacturing process of Acetic acid
- Give Mechanism of Esterification
- Discuss Strengths of Acids.
- Give manufacturing process of following acids : Formic acid, Oxalic acid, Palmitic acid & Stearic acid
- What is polynuclear compound. Write preparation, properties and uses of Naphthalene,
- write structural formula of anthracene and give one preparation and three reactions.
- What is heterocyclic compound? give synthesis and reactions of pyrrole and Furan.
- Write about nomenclature of heterocyclic comppounds.
- Give one synthesis and two uses of each: Thiophene, Pyridine and Quinoline.
- Write about the chemical properties of Thiophene, Pyridine and Quinoline.
- Compare the physical properties of Thiophene, Pyridine and Quinoline, Furan, Naphthalene, Anthracene.
- Write mechanism and four uses of these reactions: Canninzaro, Wolf Kishner and Clasien.
- What is call diazotisation? Explain with importance in different chemical manufacturing.
- Explain sandmayer reaction with mechanism.
- Compile some facts like General reaction, Mechanism, examples and applications on Curtius reaction.
- What is Baeyer Villiger oxidation reaction? Give mechanism and application.
- Give some applications of Hoffman degradation reaction with any one mechanism .
- Prepare a note with General reaction, Mechanism, examples and applications of the Michael and Dieckmann reactions.
Assignment:4
- Explain the term carbohydrates. And give classification.
- Give two chemical reactions of each: Glucose, Fructose and Starch.
- Give the conversion of any aldose to ketose and ketose to aldose.
- Give conversion of pentose to hexasose.
- Explain the manring of cane sugar from sugarcane with flow sheet.
- Define the terms i) Aminoacid ii) alpha amino acid iii) Protien
- Prepare a note on qualitative tests of proteins.
- Give any three synthesis of aminoacids.
- Give properties of aminoacids.
- What is amphoteric nature of amino acid?
- explain isoelectric point.
- Prepare a note on Primary, Secondary, Tertiary and Quartanery Structure of Protein.
- What are RNA and DNA. give types of RNA.
- Explain the importance of DNA and its structure.
Assignment:5
1. What is Drug and
Medicine?
2. What
are Antiseptics? Give an example with synthesis.
3. What
are Antimalarials? give two examples and any one synthesis.
4. Explain the term
'Antibacterials' and suggest an example with synthesis.
5. Give classification of
drug according to their therapeutic uses with examples.
6. Give synthesis and uses
of Sulphanilamide, Sulphaguanidine, Chloromycetin, and Chloroquine.
7. Write the difference
between Colour, Dyes and Pigments.
8. Give classification
of dyes based on Application and structural
representation with an example of each.
9. What are chromophore and
auxochrome explain with examples.
10. prepare a note on
theories of colour.
11. Write a note on
application of dyes and pigments.
12. give preparation of dyes:
Congo red, Malachite Green, Crystal Violet,
Alizarin, Phenolphthalein, Fluorescein, Eosin and Indigo.
13. Write a note on
occurrence and composition of crude oil.
14. Explain the distillation
of the Crude oil
15. Explain the terms:
Cracking, Knocking, Octane Number & Cetane
Number,
16. What is Synthetic petrol
write any one method of preparation of synthetic petrol.
Email address for sending the WORD and PPT files:
ocupchem2015@gmail.com
NOTE:
For Word file Size A4, font Times new roman: Title 14 Bold, Subtitle 13 normal, matters should be in 12 normal, All paragraphs should be justified, images should be sent as jpeg/png format sepratly with the mail, reactions and structures should be drawn in the ACD/ChemSketch software.Freeware link
Note: subject of the mail should be start with the Enrollment no.
(e.g 140190105--- Word file for oc&UP)
| All the students are informed to submit their open ended project as per scheduled time, in the following word file format: Format for OEP report |
Some students did not complete their submission in the subject OC&UP on the date 03/11/2015 they can do it on next day.