Assignments: Chemistry(2110001)
Assignment-1 Date of submission 30-09-2015 (on or before)
Assignment-1 Date of submission 30-09-2015 (on or before)
- Write a note on development of chemistry in science and engineering field.
- Describe the impacts of chemistry on nature and everyday life.
- What is ‘GREEN CHEMISTRY’? Write 12 principles of green chemistry and explain any two with suitable examples.
- What is ‘atom economy’? Explain in details with example or case study.
- What are the types of chemical bonding explain with examples.
- Write a note on Lewis representation of organic molecules with examples.
- Explain the classification of organic molecules.
- What is functional group? Give two examples of each functional group(any 15 groups containing organic compounds)
- What are the common sources of water? Is every source of water potable? Why?
- What are the sources of impurities in water?
- Explain the terms: Hard water, Soft water and degree of hardness.
- Why Scale and Sludge formation take place in boiler? What are the disadvantages of it?
- How will you prevent Scale and Sludge formation in boiler?
- Write a note on Caustic embrittlements.
- Describe the properties of drinking water.
- What is Break-point chlorination? Where and why it is used?
- Explain the term ‘Brackish water’? How will you carry out desalination of brackish water?
Assignment-2 Date of submission 13-10-2015 (on or before)
- What is call metal? Give difference between metal and non metal.
- What are the properties of metals: explain.
- What is an alloy? Why we make alloys?
- Draw the classification chart of alloys.
- Write a note on Non-Ferrous alloys and its industrial applications.
- Prepare a note on steel alloys and its industrial applications.
- What is corrosion? Give theories of corrosion.
- What is sacrificing anode?
- Describe the methods of protection of metals from corrosion.
- Explain the terms Inhibitors and cathodic protection.
- What is cement? Write a note on the classification of cement materials.
- Prepare a list of properties of cement and explain them.
- What are the basic row materials for manufactures of Portland Cement?
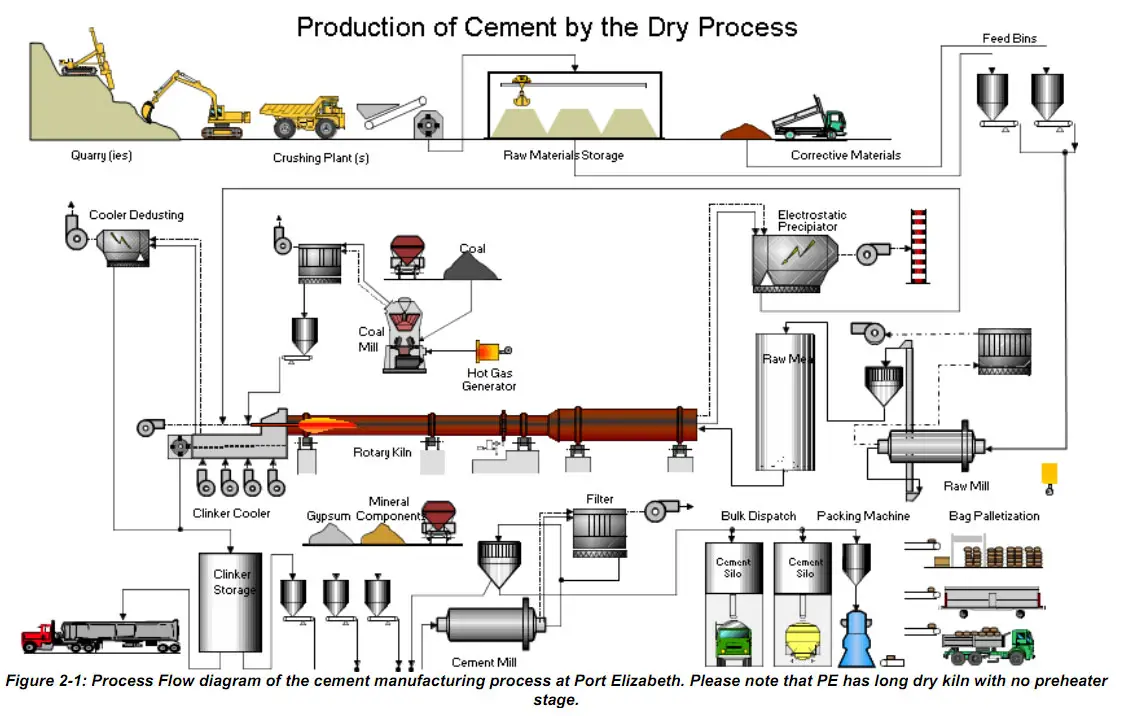
- Explain in brief manufacture of Portland cement by wet process and dry process.
- Draw the flow sheet diagram of manufacture of cement for wet process.
- Write not on setting and hardening cement.
- Write a short note on standards of cement.
- Explain the terms PCC and RCC.
- Prepare a list of uses of cement.
- Explain the environmental impact of cement industries.
Assignment-3
- Define the terms: polymer, monomer, heavy polymer, oligo polymer
- Write a note about the classification of polymers on the basis of Source, Structure, molecular forces with examples.
- Give the common mechanisms(anionic, cationic and non-ionic) of Polymerization process.
- Classify the polymers on the basis of their process of synthesis with examples.
- Write difference between thermosetting and thermoplastic polymers.
- Define rubber and give some examples.
- Classify rubber with examples.
- Give difference between natural and synthetic rubber.
- Define the term Elastomer and give properties of natural rubber.
- Explain the term vulcanization of rubber and its usefulness.
- What are biodegradable and non-biodegradable polymers give examples
- Give synthesis, properties and uses of i) PVC ii) polypropene iii) polystyrene iv)SBR
- What is call fiber? Give types with examples.
- Give propeties and uses of fibers viz. Cellulose acetic, Viscose Rayon, Nylon, Polyesters acrylic, Glass fibres, Liquid Crystals.
- Write applications of rubber.
Assignment-4
- Define Biotechnology.
- What is genetic engineering?
- Prepare a note on uses of biotechnology in different fields.
- Write six different industrial applications of enzymes.
- Define the terms : Refractory, Abrasives and Insulators.
- Write the characteristics of refractories.
- How are refractories classified?
- Write short notes on porosity and permeability of a refractory
- Prepare a note on classification, properties and uses of abrasives.
- Explain classification of Insulators
- Write properties and uses of Insulators.
Assignment-5
- Explain the following terms with their uses in chemistry i) Specific gravity ii) Melting point iii) Boiling point iv) Crystallization v) Conductivity vi) Turbidity vii) pH
- Give the difference between qualitative and quantitative analysis.
- What is spectroscopy? Give usefulness in chemical analysis.
- Define Chromatography and give applications in analytical chemistry.